It’s estimated that there are more than 400,000 Australians currently living with dementia and around 150,000 people develop dementia in Australia each year.
Without a medical breakthrough, the number of people with dementia is expected to double by 2058, according to Dementia Australia.
In order to address this impending health epidemic, Professor Michael Breakspear, psychiatrist and neuroscientist fro HMRI’s Brain Neuromodulation Research Program, successfully fought for HMRI to become one of the trial sites for an Australian Dementia Network drug trial.
HMRI was the only regional site in Australia screening patients into a trial for a drug called Lecanemab.
Beta amyloid is a naturally occurring protein that forms in everyone’s brain but it is normally cleared via the lymphatic system during sleep.
In some people, this clearing function doesn’t occur and the beta-amyloid builds up and becomes a plaque that inhibits the function of the neurons of the brain. It’s this process that leads to cognitive decline and, ultimately Alzheimer’s Disease and dementia.
Lecanemab, the drug that was trialled at HMRI, is very successful at removing amyloid from the brain.
Preliminary findings show that clearing amyloid deposits prevents Alzheimer’s Disease and dementia from developing in people who are still healthy, and it might slow cognitive decline and persevere a level of functioning in people who are symptomatic.
Lecanemab is the only drug that has been proven to be effective in treating Alzheimer’s Disease, shown to slow mental decline by 25% in people with mild dementia.
Volunteers were invited to be screened into the clinical trial. There were several prerequisites and participants had to do a number of tests to be deemed eligible.
The prerequisites were that they had to be aged between 65-80 years of age and generally healthy. They had to complete an online survey and a saliva test to determine their genetic risk profile.
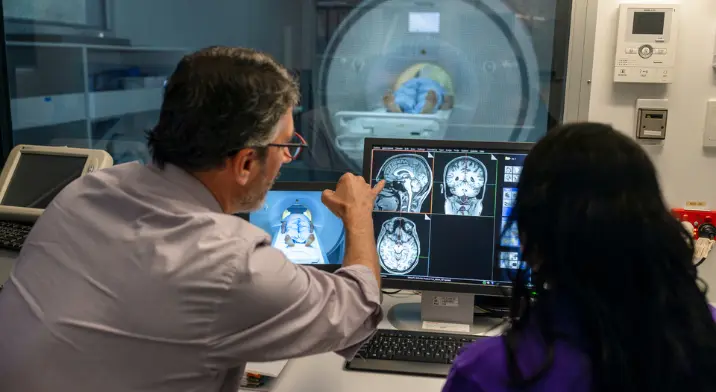
If they were accepted into the trial, they had to undertake an MRI and a PET scan to see if there was an abnormal accumulation of amyloid in their brain.
If they were found to be a high risk, they were then given fortnightly infusions of the drug or a placebo for two years. At the end of trial, the patients receiving the placebo will be treated with the Lecanemab.
400 people participated in the trial nationwide. Of 200 people screened into the Hunter trial, 60 went on to have scans, and 20 were found to have amyloid. Five of these people took part in the clinical trial.
Recruitment for this trial is now complete. The results of this trial are being considered as part of the Therapeutic Goods Administration (TGA) approval process. While already approved and available in the US, Lecanemab is expected to receive TGA approval by the end of 2024
HMRI would like to acknowledge the Traditional Custodians of the land on which we work and live, the Awabakal and Worimi peoples, and pay our respects to Elders past and present. We recognise and respect their cultural heritage and beliefs and their continued connection to their land.

Hunter Medical Research Institute
We’re taking healthy further.
Locked Bag 1000
New Lambton
NSW, Australia, 2305



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Copyright © 2024 Hunter Medical Research Institute | ABN: 27 081 436 919
Site by Marlin Communications
