Checkpoint immune drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) are offering hope to people with cancers that were once thought untreatable
Author: Dr Craig Gedye

Imagine being able to offer hope to people with cancers that were once thought untreatable. Checkpoint immune drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) are heralding this new era in cancer treatment. Some people taking these drugs can see their cancer completely disappear; there’s nothing left to see on their x-rays.
We rightly celebrate these successes, but must face the sobering truth that only a minority of people experience these dramatic benefits. Decades of research have helped us reach this point. Now scientists and doctors from Australia and around the world are working furiously to learn more about how these immune treatments work or fail.
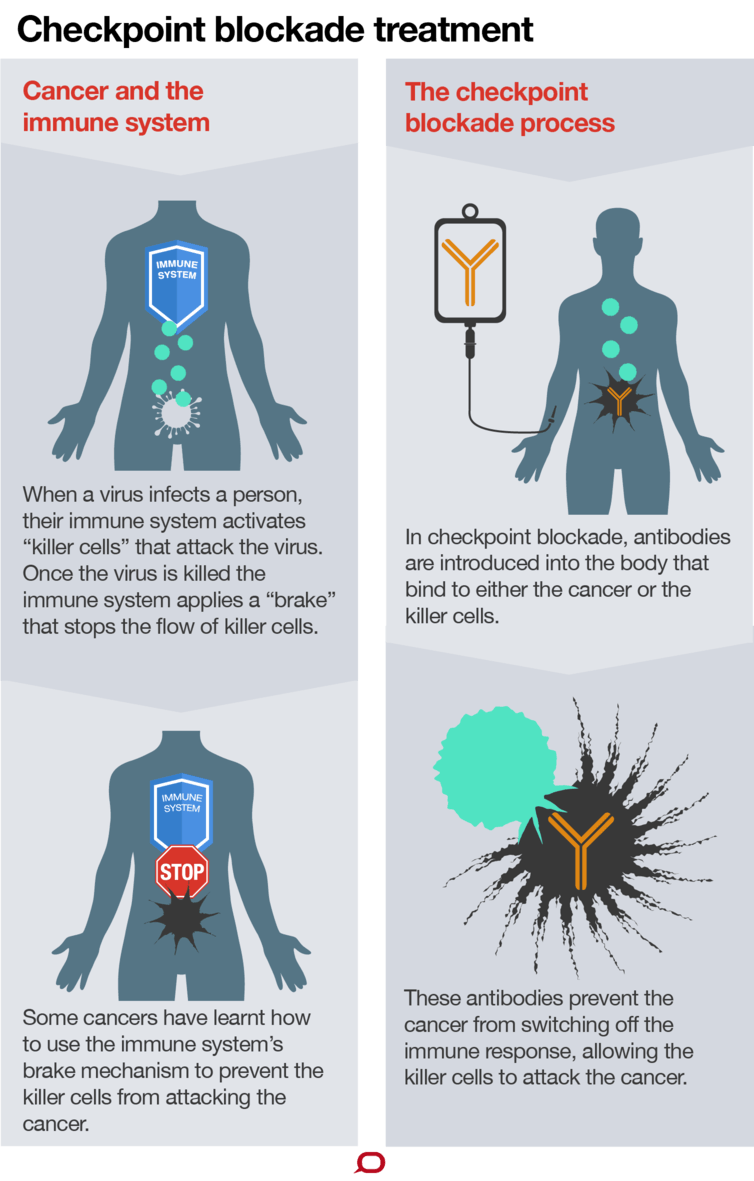
Nivolumab and pembrolizumab are checkpoint immunotherapy antibodies. They work by blocking barriers (or “checkpoints”) created by cancer cells to protect against attack from the immune system. Remove the barrier and the immune system can destroy the cancer.
The most success so far for these drugs is with melanoma, which has long been known to respond to immunotherapy. In Australia, single or combination checkpoint immunotherapy substantially helps about half of people with melanoma, and will soon be available for people with kidney and lung cancers.
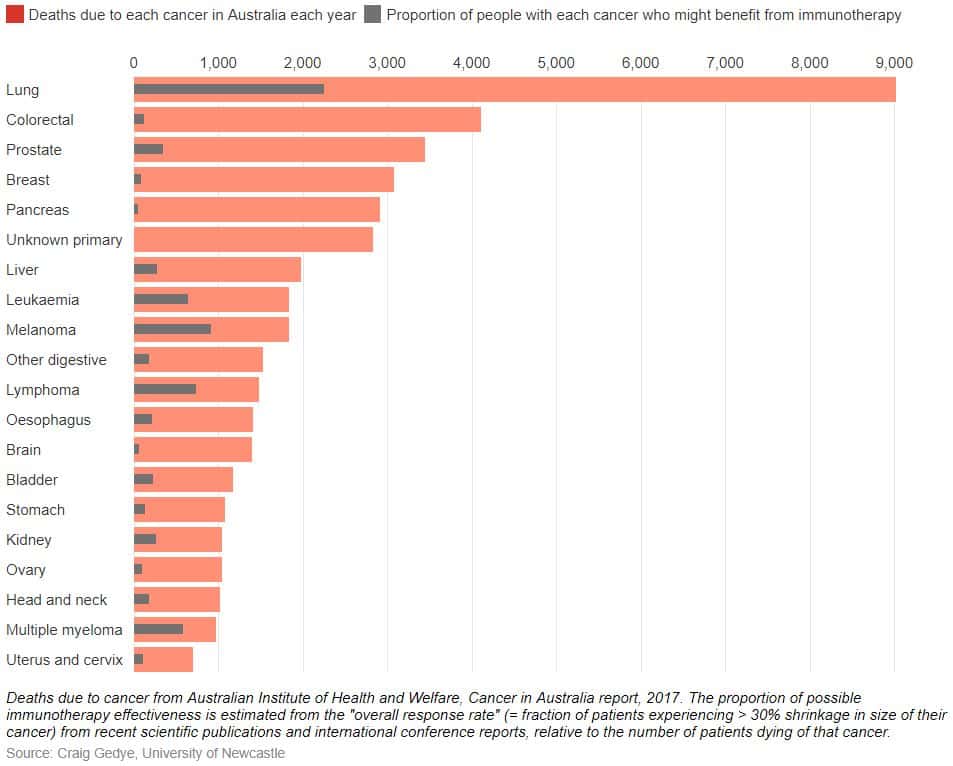
Trials continue in most types of cancer. Checkpoint immunotherapy has proven beneficial in patients with bladder cancer, head and neck cancer and Hodgkin lymphoma. A smaller proportion of people, typically around 20-30%, are helped in most of these cancers. These successes and failures start to show us how checkpoint immunotherapy works, and how it might work better.
The proportion of people who benefit varies between different types of cancer
Patients whose cancers are already under attack from immune cells are the people who seem most likely to be helped by checkpoint immunotherapy. But many patients’ cancers are devoid of immune cells – so removing checkpoints doesn’t help.
This is why the first strategy to improve checkpoint immunotherapy is to diversify and muster the immune system into tumours. Some checkpoint immunotherapy drugs (such as ipilimumab, brand name “Yervoy”) work this way. In effect they “vaccinate” the patient against their cancer, educating the immune system on how to fight the cancer, and recruiting immune cells to attack tumours.
A similar method uses modified viruses that infect and explode immune cells. These can be directly injected into cancers, drawing in immune cells to attack the cancer. This is the basis of the very first immune therapy for cancer, first used in 1896.
Finally, identifying the minority of people who naturally have immune-infiltrated cancers may identify those likely to benefit from checkpoint immunotherapy (such as aggressive breast cancer needing chemotherapy before surgery).
We may be able to identify a few people within various types of cancer most likely to benefit from checkpoint immunotherapy. For example, while checkpoint immunotherapy doesn’t help most bowel and prostate cancer patients, a small group of people whose cancers’ DNA isn’t able to repair properly have dramatic outcomes from checkpoint immunotherapy.
This lack of efficient DNA repair is called “mismatch repair deficiency”. Mismatch repair is one of the tools cells use to repair their DNA. Loss of mismatch repair leads to aggressive cancers that don’t respond to chemotherapy, but which throw up lots of targets for the immune system.
Up to a third of uterine cancers, 15% of bowel cancers, 15% of stomach cancers and perhaps 5% each of prostate, oesophageal, cervical and ovarian cancers have mismatch repair deficiency, potentially making them treatable with checkpoint immunotherapy.
Even when fully implemented, these strategies will leave many people who won’t benefit from checkpoint immunotherapy – but a huge number of new treatments, combinations and ideas are being tested in clinical trials.
Drugs that protect immune cells from toxic chemicals released by nearby cancer cells appear very promising. A myriad of new antibodies that block other immune checkpoints are in development. And we haven’t abandoned standard cancer treatments like blood-supply blocking drugs, radiotherapy or chemotherapy; these may help immunotherapy by killing enough cancer cells to recruit immune cells into tumours.
Should everyone with cancer take checkpoint immunotherapy? These drugs are safe overall, though people with autoimmune diseases (such as rheumatoid arthritis) need to be very cautious. This is because the underlying cause of checkpoint immunotherapy side effects, an overactive immune system, is very similar to the causes of autoimmune diseases.
And there is a social challenge: cost. We are privileged to have many publicly funded PBS-reimbursed cancer treatments in Australia, but drug costs are rising sharply. One solution will be to find more ways to identify the patients most likely to benefit from these drugs, so we’re not using expensive drugs to treat people for whom they won’t have an effect.
Another, perhaps complementary, strategy would be pay-for-performance – treat everyone, but only reimburse the manufacturer if the patient is helped. This might particularly assist people with rare cancers, where clinical trials are extremely hard to perform.
Checkpoint immunotherapy is a triumph – when it works. It’s important to temper our hopes with the knowledge that these promising drugs can’t yet help every person, with every cancer. But we’re working on it.
HMRI would like to acknowledge the Traditional Custodians of the land on which we work and live, the Awabakal and Worimi peoples, and pay our respects to Elders past and present. We recognise and respect their cultural heritage and beliefs and their continued connection to their land.

Hunter Medical Research Institute
We’re taking healthy further.
Locked Bag 1000
New Lambton
NSW, Australia, 2305



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Copyright © 2024 Hunter Medical Research Institute | ABN: 27 081 436 919
Site by Marlin Communications
